Information for Authors
- GENERAL
- AIM AND SCOPE
- PEER REVIEW PROCESS
- MANUSCRIPT SUBMISSION (via SCHOLAR ONE ®)
- TYPES OF MANUSCRIPTS
- PREPARATION OF MANUSCRIPTS
- CLINICAL TRIALS REGISTRATION
- PROOFING and REVISION after ACCEPTANCE
- PUBLICATION FEE
- EDITORIAL POLICY and ETHICAL CONSIDERATIONS
- ADVERTISING POLICY
- EDITORIAL OFFICE
GENERAL
Interventional Radiology, an official journal of the Japanese Society of Interventional Radiology and Asia Pacific Society of Cardiovascular and Interventional Radiology(APSCVIR), publishes original manuscripts dealing with clinical investigations, basic research and case reports in all aspects of interventional radiology, in English. Articles in Interventional Radiology follow Continuous Article publication (CAP) model, where accepted articles are published on a continuous basis.
Interventional Radiology adheres to the Recommendations for the Conduct, Reporting, Editing, and Publication of Scholarly Work in Medical Journals (http://www.icmje.org/recommendations/browse/) by the International Committee of Medical Journals Editors (ICMJE) and the Principles of Transparency and Best Practice in Scholarly Publishing (a joint statement by the Committee on Publication Ethics (COPE), the Directory of Open Access Journals (DOAJ), the World Association for Medical Editors (WAME), and the Open Access Scholarly Publishers Association (OASPA); https://doaj.org/apply/transparency/).
AIM AND SCOPE
The aims of the Interventional Radiology are to update the knowledge in interventional radiology through the publications freely available online, and to contribute to improving interventional techniques and clinical results in clinical practice. Interventional Radiology publishes peer-reviewed original research work including laboratory and clinical investigations as original research, technical notes, case reports, and pictorial reviews, and letters to the editor in the field of vascular and non-vascular interventional radiology, and interventional oncology.
PEER REVIEW PROCESS
Peer review is an important process of evaluation for any manuscript submitted to Interventional Radiology. Every article submitted for full peer review will receive a comprehensive, fair, unbiased critical assessment. The Journal employs a double-anonymized review process. This means the identities of the peer reviewers and the authors remain anonymous to each other.
The main document of the submitted manuscript should adhere to the following requirements:
- Not include the name of the affiliation anywhere in the manuscript, including the Figures and Tables.
- Refer to the authors’ previous work as that of a third person, e.g. replace “…as we have reported in our previous study19” with “as it has been reported previously19”
- Not include the references to funding sources, such as identifier of the government-related funds.
- Include acknowledgments in the title page (if applicable).
- Declare the Conflicts of Interest (COI) on the title page (if applicable).
- Include the clinical registration number in the title page (if applicable).
All submitted manuscripts will be initially reviewed by the Editor-in-Chief (EIC) of Interventional Radiology to evaluate eligibility for publication. The editors will assess the importance and originality of the research, suitability and interest to the readership of the Journal, and the validity and the quality of the manuscript. Any manuscripts that satisfy our screening criteria will generally be sent to two experts in the field of study for peer review.
The editors of the Interventional Radiology review the peer review comments and the manuscript and make all decisions on the manuscript publication, which include acceptance, major or minor revisions, and rejection based upon the reviewers’ comments. The decision letters along with the comments by the editors and reviewers will be sent to the corresponding author via e-mail.
Interventional Radiology adheres to Committee on Publication Ethics’ Ethical Guidelines for Peer Reviewers. Reviewers are not allowed to contact the authors directly before, during, or after the reviewing process to discuss any information that is presented in the manuscript. Reviewers must keep the manuscripts and information obtained strictly confidential and must not publicly discuss or disclose the contents and any other information of the manuscript to a third party. The information for the reviewers are available here.
Revised Manuscript
Manuscripts that receive a revision, authors should submit the revised manuscript by the due date instructed in the decision letter. All authors must approve every revision, correction and amendment prior to re-submission of the revised manuscript. Authors must include an anonymized, detailed point-by-point response to the reviewers’ and editors’ comments when submitting a revised manuscript.
Editors and Journal Staff as Authors
Manuscripts submitted by editors, editorial board members, or journal staff will follow the same process as outlined above. However, they are excluded from any editorial decision process of their own manuscript and have neither access to that manuscript nor any information about the review process other than what is provided in the editor’s decision letter. The editorial office will assign the paper to an editor who is not an author on the paper nor has any conflict of interest with the authors. The manuscript submitted by editors, editorial board, and journal staff of Interventional Radiology should include a statement that declares their personal conflict of interest with the Journal.
MANUSCRIPT SUBMISSION (via SCHOLAR ONE ®)
Interventional Radiology receives online submission only. Submit manuscript files electronically via the ScholarOne system (https://mc.manuscriptcentral.com/ivr) in the following order: Title page, Main Text, Tables, and Figures (≥300 dpi). The total size of the uploaded files should be within 100 MB. Upon submission, the manuscript will be automatically checked for plagiarism by the iThenticate plagiarism screening service to determine both textual overlap and manuscript originality.
TYPES OF MANUSCRIPTS
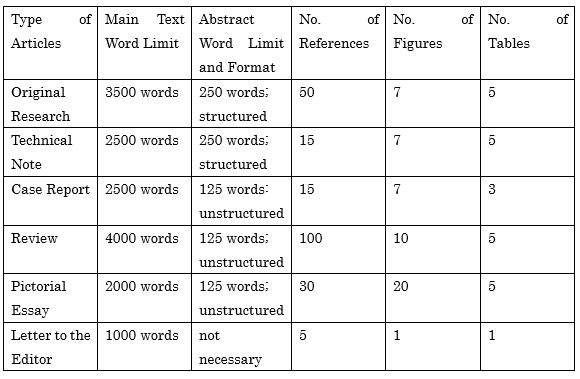
Original Research
Original Research should include a novel technique or new knowledge, which contributes to the field of interventional radiology. The method should be reasonable and described clearly. Any limitations of the work should be addressed and discussed. The conclusions should be consistent with the results obtained. A small case series with less than five (5) cases should be submitted as a “Case Report” or “Technical Note”.
The main text of Original Research should not exceed 3,500 words (the word count limit only includes main text), and the number of references is limited to 50.
Technical Note
A Technical Note should describe a novel technique in interventional radiology. The main text should not exceed 2,500 words (the word count limit only includes main text).
Case Report
A Case Report describes a single or a few cases involving rare diseases or conditions, or unique interventional techniques. The main text should not exceed 2,500 words (the word count limit only includes main text).
Review
A Review should provide a broad overview and update on a particular topic in interventional radiology. The contents of the Review should be supported by references. Original data or findings from unpublished studies should not be included in Review articles. The main text of a Review should not exceed 4,000 words (the word count limit only includes main text) and the number of references is limited to 100.
Pictorial Essay
A Pictorial Essay should consist of figures, illustrations and pictures to impart descriptive information, with minimal text. The essay should not contain new data or statistics. The main text of the Pictorial Essay should not exceed 2,000 words (the word count limit only includes main text), and the number of references is limited to ten (10).
Letter to the Editor
A Letter to the Editor is brief, constructive commentary that can be submitted in response to a recently published article in the Journal. The main text of the Letter to the Editor should not exceed 1,000 words (the word count limit only includes main text), and the number of references is limited to 5.
PREPARATION OF MANUSCRIPTS
The information provided below is based in part on “Recommendations for the Conduct, Reporting, Editing, and Publication of Scholarly work in Medical Journals,” issued by the International Committee of Medical Journal Editors (ICMJE). For any information that is not mentioned in these guidelines, authors should refer to the ICMJE Recommendations.
Manuscripts that do not follow the instructions below WILL BE RETURNED to the corresponding author for technical revision before undergoing peer review.
All manuscripts must be written in English, typed double-spaced with wide margins throughout. Line numbers and page numbers on each page are required to make it easier for reviewers to provide their comments. Incomplete or improperly prepared manuscripts will be returned to authors without review.
A title page should be prepared as a separate file from the main document. The main document should be prepared in the following order: abstract, text, references, figure legends, tables and figures. Pages must be numbered in the upper right-hand corner of each page, starting with the “Main Text” and continuing through to the references. Standard abbreviations and units should be used.
The following list of commonly used abbreviations and acronyms may be used without explanations or full-spellings:
AAA, ABI, ADC, AICA, ANCOVA, ANOVA, AVF, AVM, CBF, CBV, CCA, CI (confidence interval), CIA, CISS, CNS, Cr, CSF, CT, CTA, CTAP, CTP, CTV, DICOM, DSA, DWI, ECA, EIA, EPI, EVAR, FDA, FDG, FIESTA, FISP, FLAIR, FLASH, fMRI, FOV, FSE, Gd-DTPA, GS, GSV, HASTE, HCC, HIV, ICA, IV, MANCOVA, MCA, MDCTA, MIP, MPR, MPRAGE, MR, MRA, MRI, mRS (modified Rankin Scale), MRV, MTT, n-BCA, NCCT, NIHSS, NPO, OR (odds ratio), PACS, PET, PICA, PROPELLER, PV (portal vein), PVA, PWI, RFA, ROI, rtPA, SAH, SFA, SG, SMA, SMV, SNR, SPECT, STIR, SWI, T1WI, T2WI, TAE, TE, TEVAR, TI, TIA, TICI (Thrombolysis in Cerebral Infarction), TOF, tPA, TR, TSE, TTP, VA, VOI, VTE, Xe-CT
Define abbreviations which are not listed above at first appearance. Avoid using all abbreviations in the title and abstract.
Title Page
Please prepare a separate Microsoft Word file for the title page. The title page should include the following information:
(Note: This information will not be included in the PDF that goes to the reviewers)
- Title of the manuscript. (less than 40 words)
- Type of manuscript.
- Author names, institution information and email addresses of all authors. Telephone and fax numbers, and institution information (name and address including zip code) of corresponding author.
- Present addresses of authors who have moved since the study.
- Acknowledgment of grants, disclosures or other assistance.
- Contributions to the submitted work from each author. Please visit the ICMJE website for more information on authorship
- Conflicts of interest of each author must be stated. If there are no disclosures, that fact must be explicitly stated.
- Clinical registration number, if applicable.
- Three (3) to (5) key words for indexing.
*For a sample title page, please click here.
Please Note: As Intervention Radiology uses double-anonymized peer review, the title page should be submitted separately from the main document to ensure anonymity of the manuscript during the review process.
Abstract
Please provide a structured abstract of no more than 250 words for Original Research and Technical note), which should be structured into the following sections: Purpose, Material and Methods, Results, Conclusions.
An unstructured abstract of no more than 125 words is required for Case reports, Review articles, and Pictorial essays.
Abstract is not necessary for Letters to the editor.
Main Text
The main text of Original Research and Technical Notes should be divided into the following sections: Introduction, Material and Methods, Results, Discussion.
Case Reports should be divided into: Introduction, Case report(s), Discussion.
References
Number each reference in the order cited in the text. The authors are responsible for the accuracy of the information included in the references. Including AI-generated material as the primary source in the reference is not allowed.
Personal communications and unpublished data should not be included in the reference list but should be cited in parentheses (brackets) in the text, for example “(Jones T., personal communication)”. References should be formatted in the following order: names of authors; title; abbreviated journal title; year of publication; volume; inclusive page numbers.
If there are more than six (6) authors, list only the first three authors, followed by “et al.” Journal names should be abbreviated in the standard form as they appear in the NLM catalog. If the journals are not included in the NLM catalog, use the ISSN List of Title Word Abbreviations for standard abbreviations of journal names. If you are uncertain, please use the full journal name.
Journal articles
1. Kiyosue H, Ibukuro K, Maruno M, Tanoue S, Hongo N, Mori H. Multidetector CT anatomy of drainage routes of gastric varices: a pictorial review. Radiographics. 2013; 33: 87-100.
2. Kichikawa K, Uchida H, Yoshioka T, et al. Iliac artery stenosis and occlusion: preliminary results of treatment with Gianturco expandable metallic stents. Radiology. 1990; 177:799-802.
DOI Articles (online publication before issue publication with page numbers)
3. Inoue M, Tanaka T, Nakagawa H, Yoshioka T, Kichikawa K. Splenic vein embolization using coil anchors and prophylactic occlusion of a hepatofugal collateral for hepatic encephalopathy due to splenorenal shunt: Technical note and Literature Review. Case Rep Radiol. 2013; doi:10. 1155/2013/160653.
Book chapters
4. Tanaka T. Hybrid Interventional CT/Angio System. In: Mahnken AH, Rick J, editors. MR-guided interventions in radiology 2nd ed. *****: Springer; 2013. p.515-529.
For reference styles for other media formats, or for further details, please refer to Citing Medicine, which is available at http://www.ncbi.nlm.nih.gov/books/NBK7256/
Software
5. Mayo Foundation for Medical Education and Research. The total heart: the ultimate interactive guide to heart health [CD-ROM]. PC 1.1a version. Eagan (MN): IVI Pub.; 1993. 1 CD-ROM: sound, color, 4 3/4 in. Accompanied by: 1 manual.
Database/Internet Document
6. MeSH Database [Internet]. Bethesda (MD): National Library of Medicine (US). 2003 Apr– [cited 2011 Jul 8]. Available from: http://www.ncbi.nlm.nih.gov/mesh
Tables
Tables should be prepared in a separate file from the main document, and must be numbered in the order of appearance in the text. Each table should have a brief, informative title. A caption for each table is required. All abbreviations used in tables should be explained in the footnotes.
Figures
All images must be submitted as TIFF files. No other files are acceptable. Each figure must be cited in the text in numerical order and must be accompanied by a figure legend. Make sure to identify all annotations found in the figure in the caption. If any copyrighted materials are used, please include the relevant credit line as instructed by the original copyright holder.
- Figure requirements: dpi (dots per inch) Line
- Drawings: 800 dpi
- Halftone and color works: 300 dpi
- Minimum width: 76 mm
Annotations, e.g. arrows, should be used to indicate subtle but salient points. They should be put on a different layer to the main figure/illustration in the TIFF file to enable editing by editorial staff if necessary.
Videos
Up to two (2) article-related supplementary video files can be submitted. The format of the video files should be: .mp4, .mov, .wmv, .mpg or .avi. Each video clip should not exceed one (1) minute in length or 10MB. All narration must be in English.
Manuscript Specifications for Interventional Radiology
*Introduction, Case report(s), Discussion
CLINICAL TRIALS REGISTRATION
In accordance with ICMJE’s policy on trial registration, all clinical trials must be registered with a public trials registry before the time of first patient enrollment. ICMJE defines clinical trials as any research project that prospectively assigns people or a group of people to an intervention, with or without concurrent comparison or control groups, to study the cause-and-effect relationship between a health-related intervention and a health outcome. Health-related interventions includes but are not limited to those used to modify a biomedical or health-related outcome; examples include drugs, surgical procedures, devices, behavioral treatments, educational programs, dietary interventions, quality improvement interventions, and process-of-care changes.
Interventional Radiology requires all clinical trials to be registered with databases that are accessible to the public at no charge, open to all prospective registrants, managed by a not-for-profit organization, have a mechanism to ensure the validity of the registration data, and are electronically searchable.
Submitted manuscripts must include the unique registration number in the title page as evidence of registration. The name of the registration database must also be provided. For details regarding the required minimal registration data set, please go to the ICMJE site at http://www.icmje.org/recommendations/browse/publishing-and-editorial-issues/clinical-trial-registration.html
The Journal accepts registration from the following list of registries as well as others listed at https://www.icmje.org/about-icmje/faqs/clinical-trials-registration/:
- Clinical Trials (http://www.clinicaltrials.gov/)
- Australian New Zealand Clinical Trials Registry (http://www.anzctr.org.au/)
- ISRCTN Register (http://isrctn.org)
- Netherlands Trial Register (https://www.who.int/clinical-trials-registry-platform/network/primary-registries/netherlands-trial-registry-(ntr))
- UMIN Clinical Trials Registry (http://www.umin.ac.jp/ctr)
- EudraCT (https://eudract.ema.europa.eu/)
In reporting randomized clinical trials, authors must comply with published CONSORT guidelines. The recommended checklist must be completed and provided to the Journal at the time of manuscript submission. The recommended trial flow diagram should be presented as a figure.
Reporting Guidelines
Various reporting guidelines have been developed for different study designs. Authors are encouraged to follow published standard reporting guidelines for the study discipline.
- CONSORT for randomized clinical trials (http://www.consort-statement.org/) – this guideline is required for all randomized clinical trials, as noted above.
- CARE for case reports (http://care-statement.org/)
- STROBE for observational studies (http://strobe-statement.org/)
- PRISMA for systematic reviews and meta-analyses (http://prisma-statement.org/)
- STARD for studies of diagnostic accuracy (http://www.equator-network.org/reporting-guidelines/stard/)
- SAGER guidelines for reporting of sex and gender information (https://researchintegrityjournal.biomedcentral.com/articles/10.1186/s41073-016-0007-6)
Please access http://www.equator-network.org/ to find the guideline that is appropriate for your study.
It is extremely important than when you complete any Reporting Guideline checklist that you consider amending your manuscript to ensure your article addresses all relevant reporting criteria issues delineated in the relevant reporting checklist.
Data Sharing
The Journal encourages the authors of manuscript which includes clinical trials to share their de-identified research data including, but not limited to raw data, processed data, software, algorithms, protocols, methods, materials, study protocol, statistical analysis plan, informed consent form, clinical study report, analytic code. As required by ICMJE, all manuscripts that report the results of clinical trial must include a data sharing statement with a link to the trial registration. The statement should include the following information:
- Available types of data,
- Available documents (study protocol, statistical analysis plan, informed consent form, clinical study report, or analytic code)
- Available dates
- With whom the data are available.
- Types of analyses the authors are willing to share the data
- Method of requesting the data.
The statement is published alongside their paper.
PROOFING and REVISION after ACCEPTANCE
After the acceptance of a manuscript for publication, galley proofs will be available to the authors for corrections of minor errors such as spelling errors and omitted characters or letters. Any other corrections and revisions after the acceptance of a manuscript are not permitted unless requested by the Editorial Board of the Journal. Authors are expected to perform the proofing, as instructed by the Editorial Office. Upon completion of the proofing, authors should immediately e-mail the revised proof to the publisher. After publication, further changes, or corrections, can only be made in the form of an Erratum which will be hyperlinked to the original article.
PUBLICATION FEE
The Journal charges a fee upon acceptance of a manuscript for publication. If there is at least one (1) author who is a member of JSIR or APSCVIR, the fee for JSIR or APSCVIR member will apply:
- JSIR or APSCVIR Members: US $ 100
- Non-Members: US $ 200
EDITORIAL POLICY and ETHICAL CONSIDERATIONS
Exclusive Submission
Submitted manuscripts must be original and not have been previously published, or under consideration for publication elsewhere. Manuscripts that appear to be excessively duplicative of other published materials will be rejected. Articles that have been previously published or are being considered for publication in another journal in any language will not be accepted.
Copyright
Copyright of articles and their contents published in Interventional Radiology belong to the Japanese Society of Interventional Radiology.
Interventional Radiology is an open access journal distributed under the Creative Commons Attribution-NonCommercial 4.0 International License (https://creativecommons.org/licenses/by-nc/4.0/). Anyone may download, reuse, copy, reprint, distribute, or modify articles published in the journal for not-for-profit purposes if they cite the original authors and source properly. For for-profit or commercial use, written permission by the Editorial Board of Interventional Radiology is mandatory. The license can be found on the last page of the published PDF of the article.
Authors will be asked to sign the Copyright Transfer Agreement Form when the manuscript is accepted. All authors must read and agree to the conditions outlined in the form. The corresponding author can sign on behalf of co-authors. Manuscripts cannot be published until a signed form has been received by the Editorial Board of Interventional Radiology. A link for downloading the form will be provided in the acceptance letter. Authors should be aware that if any copyrighted or previously published materials are used in the manuscript, it is the authors’ responsibility to obtain the necessary permission prior to submitting the manuscript.
Confidentiality
All manuscript details, author information, reviewer identities, comments to the editors and the authors, and the content of the decision letter are considered privileged information and will never be disclosed to third parties.
Authorship/Contributorship
All authors listed in the manuscript must meet the following criteria of contribution described by ICMJE in the Recommendations for the Conduct, Reporting, Editing, and Publication of Scholarly work in Medical Journals.
- Substantial contributions to the conception or design of the research or the acquisition and analysis of data, and
- Drafting the work or reviewing it critically for important intellectual content, and
- Final approval of the version to be published, and
- Agreement to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Contributors who do not meet all 4 criteria for authorship above should not be listed as authors. Guest or honorary authorship is not permitted. The corresponding author must ensure that a manuscript is read and approved by all authors prior to submission.
Those who do not qualify for an authorship may be acknowledged individually or together as a group under a single heading with “Acknowledgements” the on the title page. Examples of activities that do not qualify a contributor for authorship are acquisition of funding, general supervision of a research group, or general administrative support and writing assistance, technical editing, language editing, and proofreading.
Authors should discuss, determine and (if they exist) settle any disagreements about the order of authorship before submitting their manuscript. Final author order must be established by the end of the revision phase of the peer review process. Any changes such as order, addition, and deletion of authors, between the initial manuscript submission and the final decision, should be discussed and approved by all authors. Any request for such changes must be explained for obtaining a permission for the change.
Adding, deleting, or changing the author names and their order is not permitted after the acceptance of the manuscript for publication.
Use of Artificial Intelligence (AI)-Assisted Tools/Technologies In consonance with the COPE’s position statement, WAME’s recommendations, and ICMJE’s Recommendation, Interventional Radiology does not allow artificial intelligence (AI)-assisted tools/technologies such as Large Language Models (LLMs), chatbots, or image creators to be listed as author or co-author. As described in the ICMJE, those tools cannot be responsible for the accuracy, integrity, and originality of the work, thus they do not meet the ICMJE’s criteria for authorship listed above. The authors (humans) are fully responsible for any materials of the submitted work, including the use of AI-assisted tools or technologies. AI should not be cited as an authors. Authors (humans) are also responsible for plagiarism including in text and AI-produced images. Authors must disclose, upon submission and in the Materials and Methods (or similar section), any use of AI-assisted tools or technologies in the writing of a manuscript, production of images or graphical elements of the paper, or in the collection and analysis of data.
Conflict of Interest (COI) and Sources of Funding
Authors must explicitly state whether potential conflicts of interest (COI) exist or not. This includes, but is not limited to, agreements for research support (including research funding and provision of equipment or materials), honoraria (such as lecture fees), consulting, employment, promotional fees, advisory role, stock ownership, patent/licensing fees, and any other financial, institutional or personal relationships with biotechnology manufacturers, pharmaceutical companies, or other commercial organizations that have any interest in the subject matter, materials, or process(es) discussed in the manuscript. This is applicable not only to the authors but also to the authors’ immediate family members and those who share the same livelihood. The authors must explain these companies’ role in the research. Any possible COI related to the study presented in the manuscript must be disclosed on the title page under the heading “Conflicts of Interest” using the following examples for each author: “A (author name) received honoraria from Z (entity name); B holds an advisory role in Y; C is an employee of Company X.”
If a manuscript is accepted for publication, the disclosures will be published as they appear in this section. If there are no COIs, the authors should state “The authors declare that there are no relevant conflicts of interest” on the title page.
The corresponding author must complete COI form notifying any relevant conflict of Interest for all authors. In the form, the authors must explain these companies’ role in the research.
Disclosure is not intended to prevent authors with potential conflicts of interest from contributing to Interventional Radiology.
Research Ethics
- Clinical research included in articles, which report on human subjects or materials of human origin, must comply with the provisions of the Declaration of Helsinki, and it must be mentioned that the study had been approved by the relevant institutional or national review board (IRB). If no approval from any IRB was required, that must be explicitly stated in the manuscript. Those researchers who do not have institutional or national ethics review committees should follow the principles outlined in the Declaration of Helsinki.
- Any studies involving human subjects must clearly indicate that written consent has been obtained from all patients and relevant persons (such as the parent or legal guardian) to publish the information, including photographs.
- Any data or information such as patient names, initials, hospital patient identification codes (patient IDs), specific dates, or any other information that may identify patients must not be presented anywhere in the manuscript, including the Figures and Tables. All pictures should focus on the affected areas only.
- Articles reporting on data from animal testing must indicate in the “Subjects and Methods” section the approval of the testing design by the affiliated institution’s Animal Care and Use Committee, without mentioning the name of the institution using the phrase “our affiliated institution.”
- Authors of articles reporting on new DNA sequences must furnish those data to the Gene Bank and include the accession number in the article.
Publication Misconduct
The Journal follows the recommended procedures outlined by COPE International Standards for responsible research publication for authors and editors when dealing with allegations of misconduct.
All authors are fully responsible for the originality and contents of their submitted manuscripts. All records and data presented in the manuscripts must be accurate, without any research misconducts such as fabrication, falsification, or plagiarism, or any other research or publication misconduct.
- Fabrication: Fabrication is inventing data or results of research and recording or reporting them in order to deceive people.
- Falsification: Falsification is inaccurate presentation of research results with the intention to give a false impression. This includes manipulation of research instrumentation, materials, and processes, changing, adding or omitting data, manipulating images, and omitting research results. Scientific images for publication must be minimally processed. We understand that some image processing may be necessary. Applications of adjustments, such as brightness, contrast, or color are permissible as long as these adjustments are applied to an entire image uniformly and do not selectively enhance, eliminate, or mispresent any elements in the original image, including the background.
- Plagiarism: Plagiarism is the use of another person’s ideas, processes, results, words, or theories as if they were the author’s own, without giving appropriate credit. This involves any part of the manuscript, including the figures and tables. All information and content that originate from other resources must be credited and cited, and included in the “References” section. Upon submission, the manuscript will be automatically checked for plagiarism by using plagiarism screening services or software to determine both text overlap and manuscript originality.
- Redundant or Duplicate Publication
Articles that are being considered for publication in another journal, including advanced publications such as “in-press” or “E-pub ahead of print” articles, in any language will be regarded as redundant or duplicate publication. The Journal does not accept submissions which have previously appeared on preprint servers such as bioRxiv and medRxiv, or other venues such as blogs. The author should notify the editor formally about all submissions, postings, and previous reports that could be regarded as redundant or duplicate publication of the same or similar work. Any such material must be referred to and referenced in the submitted work. Copies of such work should be included with the submission. Abstracts or posters presented at scientific meetings are not considered previously published work. - Citation Manipulation: Citation manipulation, such as inclusion of references from other publications without actually reading the cited work, or self-citing works that are irrelevant, must be avoided.
- Improper Authorship/Contributorship: All authors listed in the manuscript must meet the following criteria of contribution described by the ICMJE in the Recommendations for the Conduct, Reporting, Editing, and Publication of Scholarly work in Medical Journals. Exclusion of authors who made a definite contribution, or inclusion of individuals as authors who have not made a definite contribution to the work is not permitted. Consent to submit to the Journal must be obtained explicitly from all authors prior to submitting a manuscript.
- Salami-Slicing: “Salami-slicing” or dividing a single study into several parts to increase the quantity of submissions to other journals or the same journal is not permitted.
- Noncompliance with Local Laws and Regulations: Authors must comply with local regulations and laws if the work involves animals or human subjects, or if it involves investigational drugs, recombinant products, new devices, or any chemical materials that may be hazardous in their use.
- Copyright Infringement: All information and contents that originate from other resources must be credited and cited. If any copyrighted or previously published material, adapted, edited or otherwise, are used in the manuscript, the author must obtain permission from the copyright owner(s) prior to submitting the paper for review. Also, the authors must cite the source and indicate that permission has been received, as required by the copyright owner(s).
- Failure to Obtain IRB Approval: Clinical research included in articles, which report on human subjects or materials of human origin, must comply with the provisions of the Declaration of Helsinki, and it must be mentioned that the study has been approved by the relevant institutional or national review board (IRB). If no approval from any IRB was required, that must be explicitly stated in the manuscript.
- Author’s Undisclosed Conflict of Interest : Authors must explicitly state whether potential conflicts of interest (COI) exist or not. This includes, but is not limited to, agreements for research support (including research funding and provision of equipment or materials), honoraria (such as lecture fees), consulting, employment, promotional fees, advisory role, stock ownership, patent/licensing fees, and any other financial, institutional or personal relationships with biotechnology manufacturers, pharmaceutical companies, or other commercial organizations that have any interest in the subject matter, materials, or process(es) discussed in the manuscript. Any possible COI related to the study presented in the manuscript must be disclosed on the title page under the heading “Conflicts of Interest”
- Procedures for Handling Allegations of Publication Misconduct
Interventional Radiology follows the Committee on Publication Ethics’ (COPE) guidelines and flowcharts for handling allegations of publishing misconduct pre- and post-publication. For any information that is not mentioned in the COPE guidelines, please refer to COPE’s flowcharts.
i) Allegations of misconduct in submitted manuscripts
When editors, reviewers, authors and/or editorial staff suspect any instances of ethical misconduct during peer review, they should bring them to the attention of the EIC. The manuscript will be placed on hold. The EIC will review the case and make the preliminary assessment. If the EIC finds that an explanation from the authors is necessary, the EIC will send the corresponding author a notification, which points out the allegation and requests an explanation.
If the corresponding author does not respond and/or provide sufficient rationale for the raised concern, or the EIC is presented with evidence that establishes the ethical breach, regardless of the severity, the EIC will refer the case to the Editorial Board, which discusses the allegations, explanations, evidence, possible sanctions, and corrective actions, such as publishing an erratum, expression of concern, or retraction. Possible sanctions may include:
- official warning to the author
- immediate rejection of the manuscript
- publication of formal notice of misconduct
- formal notice to an author’s institution
- formal embargo on future contributions to the Journal.
The authors will be notified of the Editorial Board‘s decision. The authors may appeal the decision by sending an appeal letter to the Editorial Board.
ii) Complaints and Appeals
We consider complaints an opportunity to improve our peer review process, manuscript handling procedures, and management for journal publishing. All received complaints are dealt with constructively and in a timely manner. For procedures not summarized below, please refer to the Committee on Publication Ethics (COPE) Best Practice Guidelines in dealing with complains and appeals.
iii) Making a Complaint
For all allegations of misconduct related to fabrication, falsification, plagiarism, copyright or intellectual property infringement, breach of research ethics, authorship or contributorship disputes, conflicts of interest, or any other unethical conduct either pre- or post-publication, please submit a letter of complaint by e-mail to ivr_ed@kyorin.co.jp. The letter of complaint should include factual information and related evidence.
iv) Process for Dealing with Complaints
Once a letter of complaint is received, an e-mail confirming the receipt will be sent to the complainant within 3 business days (JST), with assurance that the appropriate action will be taken immediately. The received complaint will be reported to the EIC, who will refer it to the editors and other officials that are relevant to the issue. In a case of a publication ethics violation, the allegations will be investigated and the necessary decisions will be made in accordance with the COPE’s guidelines and flowcharts. The result of the investigation will be determined within 4 weeks, if possible. If this is not possible, the progress of the investigation will be sent to the complainant until the issue is resolved.
v) Appeals for Editorial Decisions
Editors of the Journal apply their best efforts to provide fair and unbiased reviews and decisions. However, if an author strongly feels that an inappropriate decision has been made by the editors, the Journal allows a single appeal of the manuscript’s editorial decision. An appeal should include the detailed information and the clear reasons for the appeal, and it should be e-mailed to ivr_ed@kyorin.co.jp. All received complaints will be forwarded to the EIC, who will refer it to the editor who handled the manuscript, or the editorial board, and they will review the appeal and determine whether any changes to the decision should be made. This may require re-review of the manuscript. The new decision made after the appeal is considered final.
vi) Post-publication Discussions
If readers have a concern on any articles published, they can begin a post-publication discussion by submitting a letter to the editor. The editor will review the letter and may ask other experts in the field to review the content. If appropriate, the editor will ask the authors of the original article to comment and publish both the original letter and the response together.
vii) Retractions
Interventional Radiology adopts the following retraction process:
- Instances requiring an investigation are brought to the attention of the EIC.
- The EIC investigates the case following the step-by-step guidelines provided in the COPE flowcharts. The EIC may contact the authors to request an explanation, which will be evaluated.
- The final decision as to whether to retract is then communicated to the author and, if necessary, any other relevant bodies, such as the author’s institution.
- The retraction statement is then posted online and published in the next available issue of the journal.
The Journal may issue retractions to alert the readers of seriously erroneous data that invalidate the conclusion of the study presented in the published article or of ethical misconduct. Retractions are published if the EIC has convincing evidence for the following cases, either as a result of ethical violations or honest error:
- The findings or data are unreliable or misleading
- Plagiarism
- Duplicate publication without permission
- Unethical research.
The retraction will include
- Information of the retracted article, such as title and authors.
- Link to the retracted article
- Reasons for retraction.
To preserve the integrity of the published record, the Journal will not remove the retracted article. It will be maintained on the platform. The PDF will be replaced with a version watermarked with the word “Retracted,” but the original text will remain accessible. A retraction notice will also be published in the next available issue.
viii) Erratum
An erratum may be issued to notify readers of important errors such as spelling, data, terms, typography, or omission, which occurred during the production process of an accepted article, which may mislead the readers. An erratum is also issued for the correction of author and contributor information.
ix) Expression of Concern
An expression of concern will be issued if the investigation of an issue concerning a published article raised suspicion but does not provide conclusive evidence, and yet the EIC feels the article contains invalid results or has strong concerns that readers should be made aware of potentially misleading information contained in the article. Also an expression of concern may be issued if the investigation requires a considerable amount of time to reach a resolution.
ADVERTISING POLICY
Interventional Radiology accepts advertising and sponsorship for its website according to the following principles:
Details
| Type of ads: | Banner |
|---|---|
| Duration: | One year (From June 1 to May 31 of following year) |
| Available number of banners: |
7 banners |
| Location of ads: | Banner space at the lower-right side of the top page of the journal https://ir-journal.jp/ |
| Price: | 700 USD (per year) |
| Ad Specification: |
|
| Inquiries: | Interventional Radiology Editorial Office c/o Kyorinsha Co., Ltd. E-mail: ivr_ed@kyorin.co.jp |
- All advertisements must clearly and prominently identify the advertiser by trademark or signature.
- Advertising must be factually accurate, and must not be misleading.
- Advertising is separate from editorial content. All advertising must be clearly identifiable as advertising and must not be confused with editorial content in format or appearance.
- Advertisement must not include content or expressions that may disturb the social order.
- Advertisement must not include the risk of defamation, infringement on privacy, damage to credit or obstruction.
- The advertisement must not use names, photographs, comments, trademarks, copyrighted works, etc. without permission from the subject.
- All advertisements are subject to approval of the Interventional Radiology , which reserves the right to reject or cancel any ad that does not meet the advertising policy of the Society.
EDITORIAL OFFICE
Questions related to submissions and editorial issues should be addressed to Interventional Radiology Assistant Managing Editor E-mail: ivr_ed@kyorin.co.jp




